
Int J Chem Res, Vol 7, Issue 4, 1-4Research Article
HEAVY METALS QUANTIFICATION AND CORRELATIVE CARCINOGENIC-RISKS EVALUATION IN SELECTED ENERGY DRINKS SOLD IN BAYELSA STATE USING ATOMIC ABSORPTION SPECTROSCOPIC TECHNIQUE
SAMUEL J. BUNU1*, DORATHY GEORGE1, DEGHINMOTEI ALFRED-UGBENBO2, BENJAMIN U. EBESHI1
1Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa, Nigeria. 2Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Bayelsa Medical University P.M.B. 178 Imgbi Road, Yenagoa, Bayelsa, Nigeria
*Corresponding author: Samuel J. Bunu; *Email: [email protected]
Received: 18 Aug 2023 Revised and Accepted: 22 Sep 2023
ABSTRACT
Objective: The study aimed to quantify the concentrations and carcinogenic-related health risks assessment of some heavy metals in selected energy drinks frequently utilized in Bayelsa State, Nigeria.
Methods: Eleven energy drinks samples were purchased from the general markets in Amassoma and Yenagoa, Bayelsa State, Nigeria, and were labeled D1–D11. The samples were digested using 10 ml of nitric acid at 120-150 ℃, and 2 ml of Perchloric acid was added after attaining room temperature, it was digested further until a clear solution was obtained, then made up to 25 ml with distilled water. The concentration of lead, cadmium, iron, and zinc were determined and quantified using Atomic Absorption Spectrophotometry (AAS), and the health-associated risks of these metals were evaluated using the standard Target Health Quotient (THQ).
Results: The EDI (Estimated Daily Intake) of lead (Pd), Cadmium (Cd), iron (Fe), and zinc (Zn) was 0.130, 0.001, 0.726, and 0.193 mg/l, respectively, all were within the World Health Organization (WHO)-acceptable range. The Chronic Daily Intake (CDI) of Pd, Cd, Fe, and Zn was obtained as 0.001 to 0.010, 5.7 x 10-5, 0.001 to 0.050, and 0.0001 to 0.010 mg/l respectively.
Conclusion: THQ for all metals analyzed was<1, the WHO acceptable limit. All the heavy metals were within acceptable THQ limits, thus posing no carcinogenic health potential risks on long-term consumption.
Keywords: Heavy metals, Carcinogenic-Risk, Energy drinks, AAS, THQ, CDI, EDI
© 2023 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2023v7i4.224. Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Energy drinks are in the category of functional beverages, which also include nutraceutical and sports drinks [1]. Nutraceutical beverages help to promote and enhance health, with concentrated extracts from teas, fruits, vegetables, or herbs as part of their constituent [2]. Caffeine, one of the main ingredients in most energy drinks has a similar chemical conformation with adenosine, thus it binds and acts as an adenosine receptor blocker in the brain [3]. The blockage of adenosine to the brain neurons inhibits the sleep-inducing biochemical effects of adenosine, resulting in the neurons firing [4]. Taurine (2-aminoethyl sulfonic acid) found naturally in the retina, skeletal, and cardiac muscle tissues, is another component of some energy drinks [5]. Taurine is associated with neuro-modulation, cellular membrane stability, and modulation of intracellular calcium levels [6].
Heavy metals are considered slow, deadly toxins that are bioaccumulated in plants and animal tissues through the natural biological cycle and sometimes from contaminated farm fertilizers or animal feeds [7, 8]. These metals disrupt normal physiological processes, leading to toxicity in several body organs. Their accumulation in different tissues is due to the gradual storage in the liver, kidney, or other organs after continuous exposure because of their low rate of excretion compared with their uptake [9]. Almost all heavy metals are unfriendly to the environment. Human absorbs these metals either via inhalation or ingestion (fig. 1) [7]. Heavy metals are not easily degraded [10].
Recent research has shown that long-term exposure to Pd in children may lead to reduced intellectual capacity [11]. Excess concentrations of Zn in the body could lead to nausea, fatigue, stomach pain, allergic reactions, and obesity [12]. High levels of Cd could cause liver, kidney, and bone diseases [13]. Cadmium has been linked to skeletal and kidney damage, GIT irritation, and pulmonary abnormalities [14].
Disorders in iron (Fe) metabolism cause anemia, and there is also a possibility of neurodegenerative diseases [15, 16]. The current study aimed to quantify the concentration of some heavy metals in selected energy drinks using AAS, and evaluate their correlative health risk with Target Health Quotient (THQ).
MATERIALS AND METHODS
Samples procurement
Eleven (11) energy drinks (labeled D1–D11) were purchased from Supermarkets in Bayelsa State. They include King_King®, Fearless®, Supa_Komando®, Monster®, Predator®, Lucozade_boost®, Bullet®, Power_horse®, Eviron®, Tiger®, and Climax®, in no particular order. Appropriate quality assurance procedures and precautions were carried out to ensure the reliability of results. The samples were completely handled to avoid any contamination. All glassware used in this work was decontaminated by washing with purified water followed by immersing for 24 h in a dilute 10% v/v nitric acid solution. Then rinsed with purified water, dried at room temperature in a dust-free environment, and stored in a clean area.
Samples digestion and atomic absorption spectrophotometric (AAS) analysis
About 20 ml of sample and 10 ml of nitric acid were transferred into a beaker and heated for 120-150 °C on a heating mantle inside the fume cupboard. Nitric acid was added from time to time to prevent dryness. After 40 min, the mixture was allowed to cool, and 2 ml of Perchloric acid was added to digest further until a clear solution was obtained. After cooling, it was transferred into a 25 ml volumetric flask and made up to volume using distilled water. The solution was filtered and transferred into 5 ml sample tubes for AAS for analysis.
The 2002 edition of an American standard test method reported by Bray et al., (2004), was followed in the digestion procedure [17]. 50 ml of the well-mixed selected sample was measured into a 150 ml beaker. 4 ml of concentrated HN03 and 2 ml of perchloric acid were added. The solution was evaporated to near dryness (low heat) and was placed at room temperature. A few drops of concentrated HN03 were continuously added and refluxed on a hot plate until a light clear residue was obtained, indicating digestion completion. About 2 ml of concentrated HN03 was added to the residue. The end solution was filtered, insoluble materials removed, made the volume up to 25 ml with distilled water, labeled appropriately, and stored in a 125 ml polypropylene bottle before aspirating the solution with the flame before AAS analysis. Atomic Absorption Spectrometer, Model D1971/4691 was used to quantify the heavy metals concentrations in the selected energy drinks, and data were analyzed using an Excel spreadsheet, 2016.
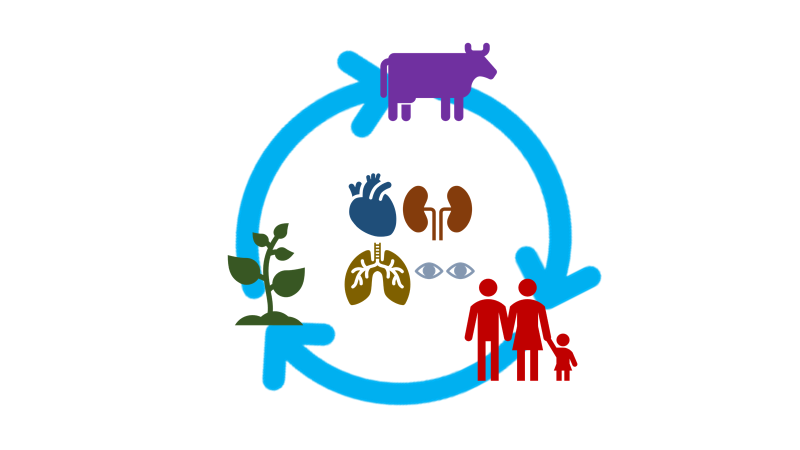
Fig. 1: Heavy metals intoxication cycle: These metals cycle in the environment is linked with the food chain as soil-plant-animal-man. Bioaccumulation affects human body organs, including the eyes, lungs, kidneys, heart, etc., [14]
RESULTS
Table 1: Heavy metal concentrations in soft drinks sample using solar thermo elemental atomic absorption spectrometer (AAS)
| Samples | The concentration of heavy metals (mg/l) | |||
| Lead (Pd) | Cadmium (Cd) | Iron (Fe) | Zinc (Zn) | |
| D1 | 0.276 | <0.002 | 0.727 | 0.162 |
| D2 | 0.147 | <0.002 | 0.907 | <0.002 |
| D3 | 0.074 | <0.002 | 0.154 | <0.002 |
| D4 | 0.073 | <0.002 | 0.919 | <0.002 |
| D5 | 0.018 | <0.002 | 0.026 | <0.002 |
| D6 | 0.166 | <0.002 | 0.226 | 0.134 |
| D7 | 0.129 | <0.002 | 1.65 | <0.002 |
| D8 | 0.037 | <0.002 | 0.113 | <0.002 |
| D9 | 0.074 | <0.002 | 0.282 | 0.047 |
| D10 | 0.147 | <0.002 | 1.27 | 0.493 |
| D11 | 0.332 | <0.002 | 1.85 | 0.332 |
| Control (H2O) | <0.009 | <0.002 | 0.026 | <0.002 |
| *WHO | 0.01 | 0.003 | 1.0-3.0 | 5.00 |
| RfD | 3.5 x 10-3 | 5 x 10-4 | 7 x 10-3 | 3 x 10-1 |
*WHO maximum permissible limit; RfD = Reference oral Dose
Table 2: Estimated daily intake (EDI) of heavy metals in selected energy drinks (mg/l)
| Sample | Lead (Pd) | Cadmium (Cd) | Iron (Fe) | Zinc (Zn) |
| D1 | 0.108 | 0.001 | 0.285 | 0.064 |
| D2 | 0.058 | 0.001 | 0.356 | 0.001 |
| D3 | 0.029 | 0.001 | 0.060 | 0.001 |
| D4 | 0.029 | 0.001 | 0.361 | 0.001 |
| D5 | 0.007 | 0.001 | 0.010 | 0.001 |
| D6 | 0.065 | 0.001 | 0.089 | 0.053 |
| D7 | 0.051 | 0.001 | 0.647 | 0.001 |
| D8 | 0.015 | 0.001 | 0.044 | 0.001 |
| D9 | 0.029 | 0.001 | 0.111 | 0.018 |
| D10 | 0.058 | 0.001 | 0.498 | 0.193 |
| D11 | 0.130 | 0.001 | 0.726 | 0.130 |
| WHO standard | 3.6µg/day/kg | 1µg/day/kg | 20.5 mg/day | 11 mg/day |
EDI = Cm x Cf x Df/Bw; Cm = Metal concentration, Cf = conversion factor (0.085), Df = Daily intake of drinks (30cl), Bw = Average body (65 kg)
Table 3: Chronic daily intake (CDI) of heavy metals in selected energy drinks (mg/l)
| Sample | Lead (Pd) | Cadmium (Cd) | Iron (Fe) | Zinc (Zn) |
| D1 | 0.008 | 5.70E-05 | 0.022 | 0.005 |
| D2 | 0.004 | 5.70E-05 | 0.027 | 0.000 |
| D3 | 0.002 | 5.70E-05 | 0.005 | 0.000 |
| D4 | 0.002 | 5.70E-05 | 0.028 | 0.000 |
| D5 | 0.001 | 5.70E-05 | 0.001 | 0.000 |
| D6 | 0.005 | 5.70E-05 | 0.007 | 0.004 |
| D7 | 0.004 | 5.70E-05 | 0.050 | 0.000 |
| D8 | 0.001 | 5.70E-05 | 0.003 | 0.000 |
| D9 | 0.002 | 5.70E-05 | 0.008 | 0.001 |
| D10 | 0.004 | 5.70E-05 | 0.038 | 0.015 |
| D11 | 0.010 | 5.70E-05 | 0.056 | 0.010 |
CDI= Cm x Edi x EFi x IR/Bw x AT; Cm =Metal concentration, ED = Exposure duration (24 y), EFi = Exposure frequency (365 d/y), IR = Intake rate (0.03 kg/person/day), AT =Average time (365 d x number of exposure years (24 y) [8,760]).
Table 4: Target health quotient (THQ) of heavy metals in selected energy drinks
| Sample | Lead (Pd) | Cadmium (Cd) | Iron (Fe) | Zinc (Zn) |
| D1 | 0.229 | 1.1E-01 | 3.143 | 0.0167 |
| D2 | 0.114 | 1.1E-01 | 3.857 | 0 |
| D3 | 0.057 | 1.1E-01 | 0.714 | 0 |
| D4 | 0.057 | 1.1E-01 | 4.000 | 0 |
| D5 | 0.029 | 1.1E-01 | 0.143 | 0 |
| D6 | 0.143 | 1.1E-01 | 1.000 | 0.013 |
| D7 | 0.114 | 1.1E-01 | 7.143 | 0 |
| D8 | 0.029 | 1.1E-01 | 0.429 | 0 |
| D9 | 0.057 | 1.1E-01 | 1.143 | 0.003 |
| D10 | 0.114 | 1.1E-01 | 5.429 | 0.05 |
| D11 | 0.286 | 1.1E-01 | 8.000 | 0.033 |
THQ =CDI/RFD; CDI =Chronic Daily Intake, RFD = Reference Oral Dose.
DISCUSSION
The concentration of Pd, Cd, Fe, and Zn was recorded in selected energy drinks and analyzed using an Atomic Absorption Spectrophotometer as listed in table 1. The concentration of Lead for all the samples of energy drinks was between 0.018 to 0.276 mg/l. This level exceeds the maximum permissible level of Pd stated by the WHO (0.01 mg/l). This result is related to another report where Pd was found in high concentrations of up to 0.447 mg/l in non-alcoholic drinks [18]. Pd causes toxicity and poisoning when body tissues are exposed to it, which can lead to high blood pressure and anemia [19]. The levels of Pd seen in the samples of energy drinks is of great interest as it can lead to toxicity of Pd in the body and other disease associated with Pd intoxication.
Spectroscopic methods are useful in the quantitation of concentrations in test samples [20]. From the AAS analysis, Cd concentration in the samples was<0.002 mg/l, below the acceptable limit of 0.003 mg/l. This concentration of Cd is not likely to cause kidney damage in consumers following long-term exposure. Low levels or high of Zinc in the body are associated with the development of prostate enlargement and human cancer [21]. The concentration of Zinc in the samples was between<0.002 to o.493 mg/l, below the permissible limit of 5.00 mg/l stated by WHO, hence not of great concern as its health benefits outweigh its toxic effects. Iron (Fe) concentration ranged from 0.026 to 1.85 mg/l, also below the acceptable limit of 3.0 mg/l as stated by WHO, hence will not have a deleterious effect on human health following long-term use by consumers.
The EDI of Pd, Cd, Fe, and Zn was estimated for all sampled energy drinks. Pd had the highest and lowest EDI of 0.130 mg/l and 0.015 mg/l respectively across the samples, which is below the WHO standard. This suggests that Pd found in the analyzed samples does not pose any threat to the health of consumers. Cd EDI was 0.001 mg/l which is below the WHO standard of 1µg/day/kg, implying that the analyzed samples are free from Cd intoxication and therefore safe for consumption. For Fe, the EDI was between 0.010 to 0.726 mg/l, below the WHO standard, while zinc was between 0.001 to 0.193 mg/l, also below the WHO standard of EDI given as 11 mg/day. Indicating that the samples are safe for consumption.
The CDI of Pd, Cd, Fe, and Zn were estimated to be 0.001 to 0.010, 5.7 x10-5, 0.001 to 0.050, and 0.0001 to 0.010 mg/l respectively. All heavy metal concentrations analyzed were within acceptable WHO standards [22].
The THQ (Target Health Quotient) is defined as the ratio of Chronic Daily Intake to toxic elements and the Reference Oral Dose which is the highest level at which no adverse health effects are expected from the consumption of food and related products. The Reference dose is specific to the trace elements being assessed. THQ is a standard parameter that describes the non-carcinogenic health risk posed by exposure to the respective toxic element. If the THQ is<1, then non-carcinogenic health effects are not expected. If, However, a THQ value of>1, suggests the possibility that adverse health effects could be experienced [23]. All the samples had THQ<1. Another similar study was conducted in Southwest Nigeria, where different heavy metals, including Mg, Zn, Pb, As, and Al; of which all the heavy metal but Aluminum was above the recommended concentration [24].
CONCLUSION
The THQ values obtained from the comprehensive study for the selected heavy metals (Pd, Cd, Fe, and Zn) were below 1, which is within the standard acceptable THQ levels, implying that there are no carcinogenic health hazards associated with the intake of the examined energy drinks. Because some chemicals have been linked to carcinogenic disposition after prolonged exposure, more eleborate nano-based quantification with larger sample sizes and hyphenated techniques should be performed before, during, and after the production of energy drinks to reduce health-related risks.
ACKNOWLEDGEMENT
The authors sincerely appreciate the Head, and all staff of the Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria.
AUTHORS CONTRIBUTIONS
Samuel J. Bunu: laboratory demonstrations, supervision, data analysis, manuscript draft, and review; Dorathy George: laboratory/field work, data generation, initial write-ups; Deghinmotei Alfred-Ugbenbo: data analysis, manuscript review; Benjamin U. Ebeshi: conception, supervision, manuscript review, and approval.
CONFLICT OF INTERESTS
There is no conflict of interest among the authors
REFERENCES
-
Mantzourani I, Terpou A, Bekatorou A, Mallouchos A, Alexopoulos A, Kimbaris A. Functional pomegranate beverage production by fermentation with a novel synbiotic L. Paracasei biocatalyst. Food Chem. 2020;308:125658. doi: 10.1016/j.foodchem.2019.125658, PMID 31655475.
-
Fereidoon Shahidi DKW. Nutraceutical beverages: an overview. American Chemical Society; 2004. p. 1-6.
-
Thomson BM, Campbell DM, Cressey P, Egan U, Horn B. Energy drink consumption and impact on caffeine risk. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2014;31(9):1476-88. doi: 10.1080/19440049.2014.940608, PMID 25010189.
-
Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem. 2011;11(8):1047-57. doi: 10.2174/156802611795347654, PMID 21401496.
-
El Idrissi A. Taurine regulation of neuroendocrine function. Adv Exp Med Biol. 2019;1155:977-85. doi: 10.1007/978-981-13-8023-5_81, PMID 31468461.
-
Pan C, Giraldo GS, Prentice H, Wu JY. Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J Biomed Sci. 2010;17Suppl 1:S17. doi: 10.1186/1423-0127-17-S1-S17, PMID 20804591.
-
Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9):e04691. doi: 10.1016/j.heliyon.2020.e04691, PMID 32964150.
-
Balali Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. 2021;12:643972. doi: 10.3389/fphar.2021.643972, PMID 33927623.
-
Witkowska D, Słowik J, Chilicka K. Heavy metals and human health: possible exposure pathways and the competition for protein binding sites. Molecules. 2021;26(19). doi: 10.3390/molecules26196060, PMID 34641604.
-
Algasham H, Farooq H, Uzun C, Skinner Ramos S, Bernussi AA, Grave de Peralta L. Scanning diffracted-light photography using white-light and thermal radiation sources. Appl Opt. 2018;57(34):9997-10003. doi: 10.1364/AO.57.009997, PMID 30645261.
-
RC, AM Menna. Symptoms of lead poisoning. Blood Disorders; 2021.
-
Fosmire GJ. Zinc toxicity. Am J Clin Nutr. 1990;51(2):225-7. doi: 10.1093/ajcn/51.2.225, PMID 2407097.
-
Izah SC, Inyang IR, Angaye TCN, Okowa IP. A review of heavy metal concentration and potential health implications of beverages consumed in Nigeria. Toxics. 2016;5(1). doi: 10.3390/toxics5010001, PMID 29051433.
-
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. 2012;101:133-64.
-
Horiguchi H, Oguma E, Kayama F. Cadmium induces anemia through interdependent progress of hemolysis, body iron accumulation, and insufficient erythropoietin production in rats. Toxicol Sci. 2011;122(1):198-210. doi: 10.1093/toxsci/kfr100, PMID 21540277.
-
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60-72. doi: 10.2478/intox-2014-0009, PMID 26109881.
-
Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537-43. doi: 10.1093/ajcn/79.4.537, PMID 15051594.
-
Ball DJ, Beierschmitt WP. Permitted daily exposure values: application considerations in toxicological risk assessments. Int J Toxicol. 2020;39(6):577-85. doi: 10.1177/1091581820946746, PMID 32794434.
-
Janus J, Moerschel SK. Evaluation of anemia in children. Am Fam Physician. 2010;81(12):1462-71. PMID 20540485.
-
Vaikosen EN, Bunu SJ, Dode E, Efidi RB. Spectrophotometric fingerprinting and chemical determination of streptomycin, amikacin, neomycin, and gentamycin sulphate by condensing with Ninhydrin reagent. Int J Chem Res. 2023;7:5-10. doi: 10.22159/ijcr.2023v7i3.221.
-
Ho E, Song Y. Zinc and prostatic cancer. Curr Opin Clin Nutr Metab Care. 2009;12(6):640-5. doi: 10.1097/MCO.0b013e32833106ee, PMID 19684515.
-
Perkins DN, Brune Drisse MN, Nxele T, Sly PD. E-waste: a global hazard. Ann Glob Health. 2014;80(4):286-95. doi: 10.1016/j.aogh.2014.10.001, PMID 25459330.
-
Aendo P, Thongyuan S, Songserm T, Tulayakul P. Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci Total Environ. 2019;689:215-22. doi: 10.1016/j.scitotenv.2019.06.414, PMID 31271987.
-
Bunu SJ, Ebeshi UB, HFK, Kashimawo AJ, Vaikosen EN, Itodo CB. Atomic absorption spectroscopic (AAS) analysis of heavy metals and health risks assessment of some common energy drinks. Pharmacology and Toxicology of Natural Medicines. 2023;3:1-9.