Int J Chem Res, Vol 8, Issue 4, 12-17Research Article
THE INDUSTRIAL IMPORTANCE OF TECHNOLOGY TRANSFER FOR ANALYTICAL METHOD DEVELOPMENT AND VALIDATION–APPLICATION TO VILAZODONE HYDROCHLORIDE DOSAGE FORM
PULAGURTHA BHASKARARAOa,b*, MATTA SARIKAa,b, CHITRA LAKSHMI MADHAVIa,b, MOHAN GUPTA KOLLIPARAc, GUNTUR PRASHANTHIa,b
aAditya Pharmacy College, Surampalem-533437, Andhra Pradesh, India. bJawaharlal Nehru Technological University Kakinada, Kakinada, Andhra Pradesh, India. CMedrich Limited, M. S. Nagar, Bengaluru, Karnataka, India
*Corresponding author: Pulagurtha Bhaskararao; *Email: [email protected]
Received: 15 Jul 2024 Revised and Accepted: 20 Aug 2024
ABSTRACT
Objective: The research aims to create a fast, economical, and efficient tech transfer method for analyzing bulk materials and pharmaceutical dosage forms.
Methods: The analysis was performed using an Acquity UPLC BEH C18 analytical column (130 Å, 1.7 µm, 3 mm x 100 mm) with detection of the analyte at 238 nm. The mobile phase flow rate was optimized at 0.3 ml/min, and an injection volume of 0.5 µl** was used. The isocratic mobile phase consisted of a blend of buffer (0.1% OPA) and acetonitrile at a ratio of 40:60% v/v.
Results: All validation parameters of the analytical method were acceptable. The analyte eluted at 3.08 min with satisfactory theoretical plates and a tailing factor. The recovery of the analyte was 99-100%, and the method's precision was 0.59 and 0.72% RSD. The technique showed no interference, indicating specificity. The linearity was demonstrated over the 20-60 µg/ml range, with a regression coefficient of 0.999.
Conclusion: The developed method is cost-effective, specific with minimal interference, and time-saving. It also reduces solvent consumption for quality control analysis of vilazodone hydrochloride samples. The method demonstrates good linearity, robustness, and accuracy.
Keywords: Vilazodone HCl, UPLC, ICH, Tech transfer, Validation
© 2024 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2024v8i4.235 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Vilazodone hydrochloride is the most common oral medicament and possesses potent and selective activities as a serotonin reuptake inhibitor an agent that acts as a potent, selective serotonin reuptake inhibitor and a partial agonist of the 5-HT1A receptor used for treating major depressive disorder caused by chemical changes in the brain triggered by life events or illness and the agent binds with enzyme allosterically. The drug vilazodone HCl was approved by the FDA in 2011[1] and the molecule was developed by Allegran with the trade name VIBRID. According to pharmacokinetics, it has good bioavailability with fatty and light food particles. Chemically5-[4-[4-(5-cyano-1-indol-3-butyl] piperazin-1-benzofuran-2-carboxamide; hydrochloride. The structure of the compound is given (fig. 1). The molecular mass is 477.9 g/mol-1and the Pka value with 7.1weak base property after the partition coefficient between n-octanol and water study.
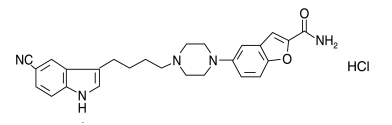
Fig. 1: Chemical moiety of vilazodone hydrochloride
Several analytical methods have been reported, which include HPLC-MS/MS [2-6], UPLC-MS/MS [7, 9], UFLC [8], HPTLC [15], HPLC [10-14], and spectrophotometric [13-20].
Previous developments in analytical methods for vilazodone hydrochloride have been criticized for their lengthy and tedious processes, as well as their insufficient sensitivity. Therefore, there is a need to develop an enhanced analytical method for vilazodone hydrochloride that offers improved sensitivity and efficiency while meeting the necessary criteria for accuracy.
In the current context of research work, the new analytical technique for vilazodone hydrochloride was developed and validated utilizing a sophisticated chromatographic system UPLC (Ultra Performance liquid chromatography) system because the system has many advantages among the conventional methods when compared to other factors like analysis run time and usage and consumption of solvent and withstanding high back pressure without experiencing any undesirable detrimental effects in the analytical column for being analyte separation and no sudden pressure development into the system. The proposed method was validated as per ICH guidelines.
MATERIALS AND METHODS
Materials and chemical reagents
Vilazodone Hydrochloride was provided as a complimentary sample by Dr. Reddy’s Labs, located in Visakhapatnam, India. Milli-Q water was used for the study and prepared using the Milli-Q Integral water purification system. All chemicals and reagents utilized for method development were sourced in analytical reagent grade without additional purification steps.
Instrumentation
The Make Waters Aquity UPLC 2695 module with binary pumps setting UV detector with autosampler. Using the Waters Empower 2 software tool, data from experimental methodology is integrated into the separation technique.
Methods
Chromatographic conditions
The method development was carried out through a calibrated UPLC apparatus comprising an Acquity UPLC binary solvent manager equipped with an Acquity automatic sampler with a sample cooling facility for samples and a UV detector compassed from Waters (Waters Inc.). Empower software is used for data analysis and data integrity. Selected the analytical column for the separation of analyte with Aqcuity UPLC BEH C18 (130 Aͦ, 1.7µm, 3 mm X 100 mm) and analyte detected at 238 nm. The flow rate by mobile phase was optimized as 0.3 ml/min and injection volume-0.5µl was employed. An isocratic mixture of mobile phase contains buffer (0.1% Orthophosphoric acid) and acetonitrile in the ratio 40:60 % v/v. A drastic change occurred and was observed at 3.05 min between a stationary phase and analyte being of interest due to the ionization state [8]. The run time of analysis was completed in about 6 min using isocratic mode operation under ambient temperature conditions along with stabilized pressure.
Preparation of standard solution and calibration curve
Transferred 25 mg API into 25 ml volumetric flask and dissolved using methanol as solvent and the concentration of solution is 1 mg/ml. Further, 100µg/ml was prepared from the same resultant solution. Appropriate standard dilutions for the methodology were prepared.
Preparation of pharmaceutical solid dosage form
Accurately weighed content amount of 20 VIBRID tablets powder corresponding to 20 mg relinquished into a 100 ml graduated flask. 1/3rd of the dilutant was added, and the flask contents were vortexed for 30 min to liberate the free form of API from the placebo. The volume of the flask was filled up to 100 ml with diluent, mixed well to get a clear solution, and filtered through the PVDF membrane to retain the placebo material into the membrane. Filtrate is used for analysis. The concentration of the prepared tablet solution was 100 µg/ml.
RESULTS AND DISCUSSION
Method development
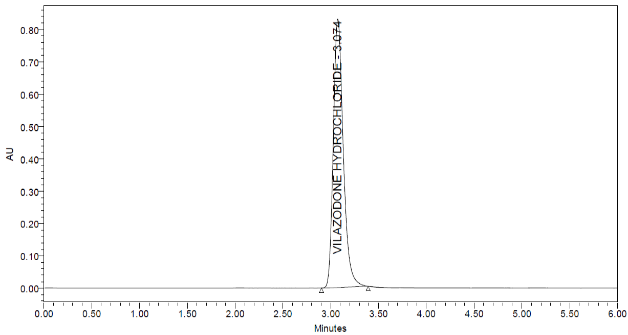
We conducted various trials with pH and change of mobile phase composition. To get a fine and specific method. The optimized chromatogram is given in (fig. 2).
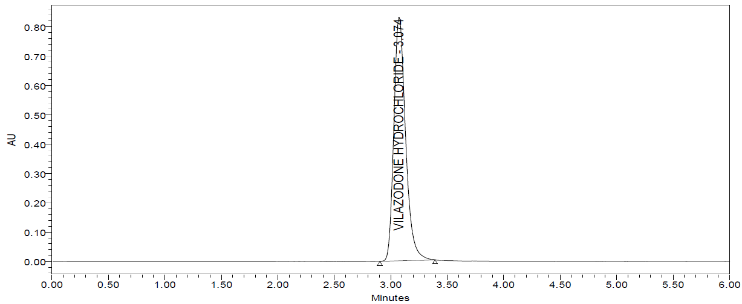
Fig. 2: Optimized typical vilazodone HCl chromatogram
Method validation
The optimized and developed method has been evaluated under the guidelines outlined in ICH Q2 (R1) from 2005.
The regulatory body (ICH) importance in the pharmaceutical industry (Technology transfer)
The objective of this regulatory body is to ensure safe, effective, and high-quality medicinal agent’s development. The key elements of the method, specifically accuracy, precision, and limit of quantification, play a crucial role in technology transfer, along with specificity, linearity, and LOD. However, the limit of quantification (LOQ) is a very important parameter in the method development of API-related substances or impurities. During the long-term stability regular evaluation of product-related parameters by performing the following: dissolution, blend assay, related substances (RS) or impurity quantification, and assay quantification of the finished product, the purity of the sample and quantification recovery has been decreased, which resulted in not following the expected trend analysis. This is due to the impurity level exceeding the specified limit as per ICH and other regulatory bodies.
The limits of impurity noticed due to various reactions and other factors within the molecule are affected by exposure to climatic conditions such as high humidity, high temperature, and crystallinity during storage. Afterward, impurity must be qualified for the effect of which is safe or unsafe in the formulation. Numerous articles have been published by academics, encompassing a wide range of work that cannot be accurately defined by method developers in terms of analytical accuracy, precision, and quantification limits. The importance of this data is evident during technology transfer and in ensuring reproducible data is submitted to the USFDA when the product is launched globally. The accuracy parameter plays a critical role in the recovery of the API amount, as well as the placebo material, across the entire range of dosages. Additionally, this parameter provides insight into the compatibility or non-compatibility of products with excipients for achieving good recovery amounts. The method's precision was assessed using inter- and intra-laboratory facilities, and the reproducibility and stability of the method were verified by injecting bracketing standards. Through the analysis process, good reproducibility data was obtained for homogeneous samples via multiple injections. The method's specificity is indicated by the absence of interference during separation. Several methods have reported stability-indicating profiles without being exposed to light. When submitting the product development report to the USFDA, the agency will seek reproducibility of data and integrity before granting permission for global product distribution.
System suitability
The UPLC system was evaluated after calibration. The system's operation was confirmed by applying all routine system setup conditions, including purging and system pressure management, and ensuring the mobile phase supply channel lines, infrits, and detector were functioning properly. Next, six replicate samples were injected into the system to obtain chromatogram results. Equipment testing was conducted using USP 24 and NF19 standards. The recorded chromatogram results are provided (table 1).
Table 1: System suitability results
| Name of the drug | Duration (Rt) (min) | Area (AU) RSD | Column efficiency plates | Symmetry factor |
| Vilazodone HCl | 3.08 ±0.13 | 6153758±0.21 | 4282 | 1.28 |
| Accepted limits [22,23,24] | RSD NMT 2 | RSD NMT 2 | >2000 | NMT 2 |
Rt = Retention time, RSD = Relative Standard Deviation, NMT = Not More Than. N=6
Specificity
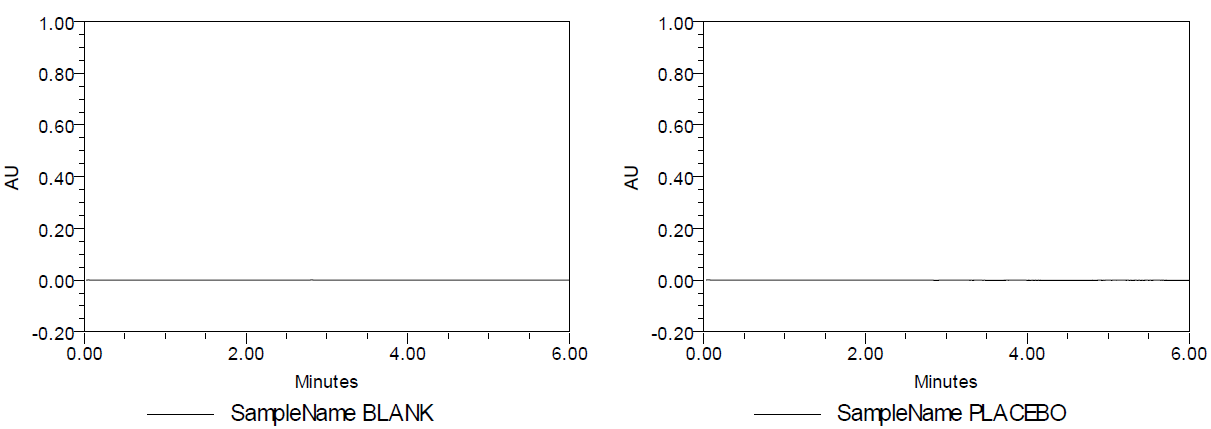
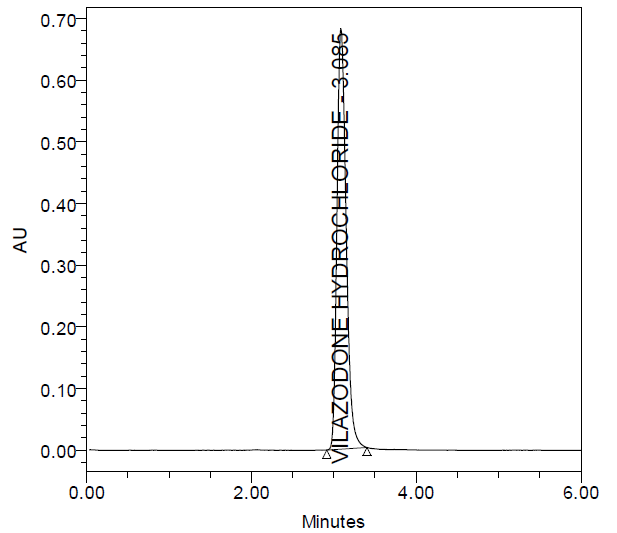
The specificity parameter provides information about the analyte being assessed unequivocally in the presence of other components [8]. The generated data has been represented (fig. 3).
A |
B |
C |
D |
Fig. 3: A. Blank B. Placebo C. Formulated product chromatogram and D. Standard
Accuracy
The accuracy of the analytical procedure was determined by assessing the consistency or agreement between acceptable values, either a conventional true value or accepted reference value, using the standard addition method [11]. Accuracy was evaluated by adding known amounts of analyte (spiked) into a pre-analyzed tablet sample containing pure vilazodone hydrochloride at 50%, 100%, and 150% levels, and the samples were injected in triplicate at each level and then calculating their % recovery at each level. The method demonstrated high accuracy and no interference with the formulated drug product. The results are presented (table 2).
Table 2: Findings from the accuracy
| Spiked level | Sample area | Amount added | Amount found | % Recovery | % Mean | % RSD |
| 50% | 3040980 | 19.80 | 19.59 | 99 | 99% | 0.58% |
| 3015257 | 19.80 | 19.42 | 98 | |||
| 3074516 | 19.80 | 19.80 | 100 | |||
| 100% | 6121063 | 39.60 | 39.43 | 100 | 100% | |
| 6127357 | 39.60 | 39.47 | 100 | |||
| 6136915 | 39.60 | 39.53 | 100 | |||
| 150% | 9251931 | 59.40 | 59.60 | 100 | 100% | |
| 9240771 | 59.40 | 59.40 | 100 | |||
| 9292355 | 59.40 | 59.40 | 101 |
*mean area of three determinations.
Top of Form
Bottom of Form
Precision
Repeatability
The precision of the proposed method was investigated using homogeneous samples, and the degree of scatter, or proximity to agreement, between several measurements was measured under specified conditions [20]. The known concentration of standard drug solution is injected as six replicates on the same day and subsequently on the following day using the same column. Calculated and summarised with % RSD value against peak area response. The outcomes are given (table 3).
Table 3: Findings of reproducibility and within-day variability
| Variable | % RSD for VLZ response (n=6) |
| Repeatability | 0.59±0.41 |
| Intermediate precision | 0.72±0.28 |
n= six determinations, mean±SEM
Top of Form
Bottom of Form
Linearity and range
The analyte was tested to see if its test results were directly proportional to its concentration in the sample, ranging from 20 to 60 µg/ml. The linearity (R2) was determined using regression analysis of the calibration curves, and the results are summarized (table 4). The graph plotted by taking concentration on the x-axis and peak response on the y-axis showed good linearity within the 20-60 µg/ml. (fig 4). The formula for the regression coefficient is given below.
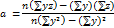
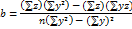
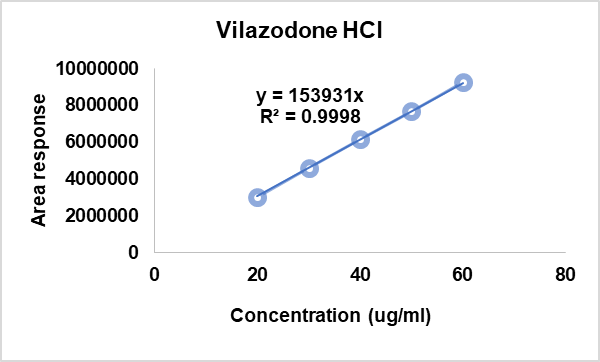
Fig. 4: Linearity curve
Detection of limit
The lowest statistically detectable analyte concentration can be determined with a signal-to-noise ratio of 3:1, and the LOD concentration is 0.249 µg/ml [15]. The chromatogram is shown below (fig. 5).
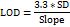
Quantification limit
The lowest detectable concentration of analyte in a sample, with a signal-to-noise ratio of 10:1 using the LOQ, is 0.075µg/ml [15]. The chromatogram is shown (fig. 5).

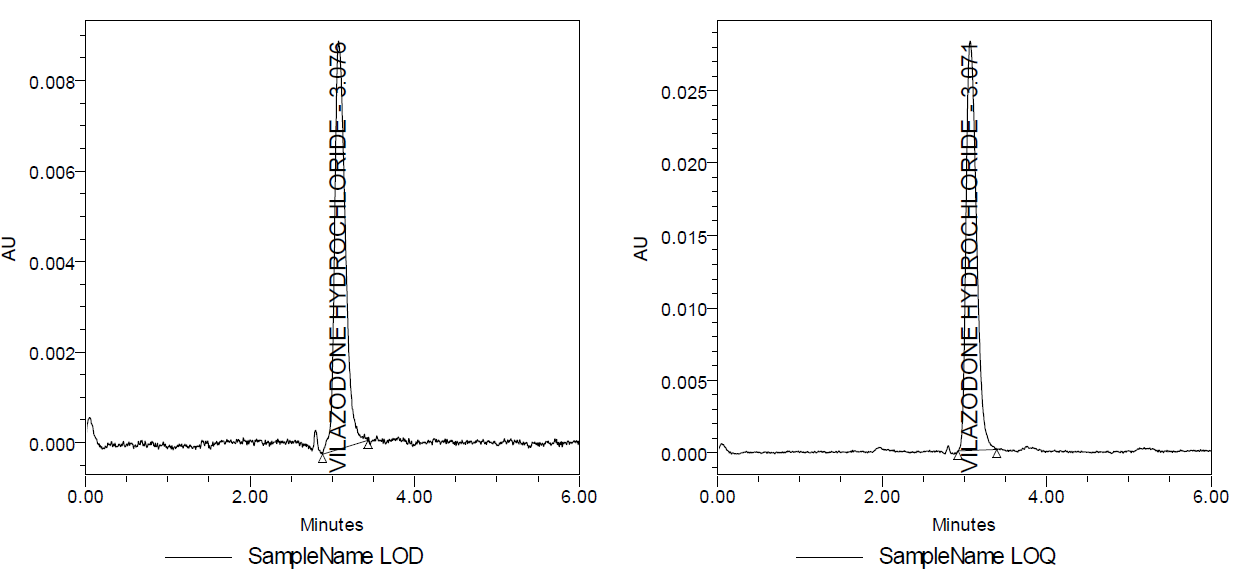
Fig. 5: LOD and LOQ chromatograms
Top of Form
Bottom of Form
Robustness
An analytical procedure's robustness is a measure of its ability to withstand slight, intentional changes in method parameters and demonstrate reliability under typical use [11]. Variables are presented (table 5).
Table 5: Parameters variation
| Parameter | Mobile phase variation | |||||
| Variation | +10% | -10% | ||||
| Vilazodone HCl | Separated time | Analyte response | Tailing factor | Separated time | Analyte response | Tailing factor |
| 3.87 | 6815339 | 1.26 | 2.54 | 4927377 | 1.41 | |
| Temperature variation | ||||||
| 3.86 | 7087954 | 1.26 | 2.54 | 5043794 | 1.44 | |
*Three determinations with±10% of temperature and mobile phase variations
CONCLUSION
The method we created has demonstrated excellent accuracy, repeatability, and sensitivity for routine analysis during tech transfer. In this method, the choice of organic modifier and aqueous solvent played a crucial role. Particularly, they had a noticeable effect when the analyte was separated by UPLC BEH C18 (130 A°, 1.7 µm, 3 mm x 100 mm) over a short period. An illumination study of at least 1.2 x 10^6 lux hours reveals the molecule's characteristics. Future work will benefit from the conclusion drawn from this study.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Work design by Bhaskara rao, Plan and Execution by Mohan Gupta, drafting article by Sarika, Lakshmi Madhavi, and Prashanthi. Finally, all authors reviewed.
CONFLICT OF INTERESTS
The authors affirm that there are no conflicts of interest to disclose.Top of FormBottom of Form
REFERENCES
Drug information. Available from: https://www.drugs.com/nda/vilazodone_100524.html
Ghosh B, Mandal P, Chakraborty S, Bera R, Saha C, Poddar S. Determination and quantitation of benzofuran indole and piperazine containing selective serotonin reuptake inhibitor vilazodone hydrochloride in human plasma by LC-ESI-MS/MS with an application to pharmacokinetic study under the framework of bioequivalence study. JAPLR. 2021;10(1):1-12. doi: 10.15406/japlr.2021.10.00361.
Anna P, Karol W, Malgorrzata SM, Bouguslaw B, Hanna KJ, Jacek G. Determination of venlafaxine vilazodone and their main active metabolites in human serum by HPLC-DAD and HPLC-MS. Acta Polomiae Pharmaceutica Drug Res. 2017;74(3):765-75. PMID 29513945.
Zeng LL, Sun LL, Zou Q, Zhou F, Wei P, Ouyang PK. Bioavailability comparison of a new form of vilazodone XVII to IV in beagles using liquid chromatography-mass spectrometry. Biomed Chromatogr. 2014;28(12):1738-43. doi: 10.1002/bmc.3215, PMID 24853720.
Wenwen S, Xiaojing Y, Wenhong Y, YI J, Xinyi L, Xiangjun W. A validated LC-MS/MS method for the rapid quantification of vilazodone in rat plasma: application to a pharmacokinetic study. J Pharm Biomed Anal. 2014 Sep;98:228-34. doi: 10.1016/j.jpba.2014.05.034, PMID 24937809.
Kalariya PD, Talluri MV, Ragampeta S. Experimental design approach for selective separation of vilazodone Hcl and its degradants by LC-PDA and characterization of major degradants by LC/QTOF–MS/MS. Chromatographia. 2014;77(19-20):1299-313. doi: 10.1007/s10337-014-2739-0.
Nikhil A, Amit M. Ultrafast bioanalytical assay for vilazodone quantification in human plasma using vilazodone D8 internal standard by UPLC-ms/ms. Res Square. 2022;1:1-12. doi: 10.21203/rs.3.rs-2316674/v1.
Suman SP, Venkata Varaha Ravi Kumar B, Sarwar B. Development and validation of a stability-indicating liquid chromatographic method for estimating vilazodone hydrochloride in pharmaceutical dosage form using quality by design. J Chromatogr Sci. 2016;54(10):1713-22. doi: 10.1093/chromsci/bmw127, PMID 27601040.
El Bagary R, Hashem H, Fouad M, Tarek S. UPLC-MS-MS method for the determination of vilazodone in human plasma: application to a pharmacokinetic study. J Chromatogr Sci. 2016;54(8):1365-72. doi: 10.1093/chromsci/bmw084, PMID 27209054.
Bipasha B, Uma Shankar M, Sudhir Kumar S. Development and validation of stability indicating RP-HPLC method for the analysis of vilazodone in bulk form and marketed pharmaceutical dosage form. RJPT. 2023;16(9):4319-24. doi: 10.52711/0974-360X.2023.00707.
Chaudhari YJ, Lokhande RS, Yadav RR. Stability indicating method development validation and forced degradation study for vilazodone hydrochloride api. Orient J Chem. 2021;37(1):204-12. doi: 10.13005/ojc/370128.
Yadav N, Anju G. New high performance liquid chromatographic method for determination of vilazodone Hcl in pharmaceutical dosage form. Asia Jour Rese Chem. 2018 Jan;11(2)427. doi: 10.5958/0974-4150.2018.00078.0.
Athavia BA, Dedania ZR, Dedania RR, Swamy SM, Prajapati CB. Stability indicating HPLC method for determination of vilazodone hydrochloride. Int J Curr Pharm Sci. 2017 Jul;9(4):123. doi: 10.22159/ijcpr.2017v9i4.20975.
Venkatasubbaih G, Devika GS, Salibai R, Hemalatha K, Gopala Krishna SV. Determination of vilazodine in pharmaceutical formulations by HPLC method. J Glob Trends Pharm Sci. 2014;5(4):2261-4.
Somsubrah G, Venkatesh S, Ravi Kumar BV. Development of stability indicating RP-HPLC method and validation for the estimation of vilazodone hydrochloride. Int J Pharm Tech Res. 2015;7(1):204-11.
Agarwal Anshu A, Damle Mrinalini C. Development and validation of stability indicating HPTLC method for estimation of vilazodone hydrochloride. Int J Pharm Res Scholars. 2015;4(1):262-8.
Derayea SM, Elhamdy HA, Badr El Din KM, Oraby M. Novel spectrofluorometric approach for assessing vilazodone by blocking photoinduced electron transfer: analytical performance and greenness blueness evaluation. RSC Adv. 2024;14(6):4065-73. doi: 10.1039/d3ra08034j, PMID 38288155.
Sartini I, Salvadori M, Lebkowska Wieruszewska B, Poapolathep A, Giorgi M. Analytical method validation of vilazodone with spectrofluorimetric detection in rabbit plasma. Am J Anim Vet Sci. 2019;14(1):50-6. doi: 10.3844/ajavsp.2019.50.56.
Rudram Devi G, G Saravanan, Mohammad Yunoos, MV Sai Krishna. A simple validated UV spectrophotometric method for the estimation of vilazodone hydrochloride in pure and marketed formulations. Indian J Res Pharm Biotech. 2017;5(2)164-7.
Nita Y, Goyal A. A validated spectrophotometric method for determination of vilazodone hydrochloride in pharmaceutical dosage form. IJCPR. 2017;9(1):132-5.
Sagar Suman P, Kumar VVR B. Spectrophotometric quantitation of vilazodone hydrochloride in pharmaceutical dosage form using quality by design approach. Malays J Anal Sci. 2015;19(5):920-9.
International conference on the harmonization. ICH harmonized tripartite guidelines. Stab Test New Drug Subst Prod. Vol. Q1A; 2003. p. R2.
International conference on harmonization. ICH harmonized tripartite guidelines. Validation of analytical procedures: text and methodology. Vol. Q2; 2005. p. R1.
The United States Pharmacopoeia 34, the national formulary 29; the US Pharmacopeial Convention. Rockville. 2011;2445:3782-4608.