
Int J Chem Res, Vol 9, Issue 3, 16-21Research Article
SOLUTE-SOLVENT INTERACTIONS OF D-(+)-MALTOSE MONOHYDRATE IN AQUEOUS SODIUM SACCHARIN AT T= (298.15-313.15) K: VOLUMETRIC AND VISCOMETRIC APPROACH
PRAJAKTA JONDHALE1 , VALMIK JONDHALE*
, VALMIK JONDHALE*
1Department of Chemistry, S. N. Arts, D. J. Malpani Commerce, and B. N. Sarada Science College (Autonomous) Sangamner-422605, Maharashtra, India. *Department of Chemistry, G. E. Society’s RNC Arts, JDB Commerce and NSC Science College, Nashik Road, Nashik-422101, Maharashtra, India
*Corresponding author: Valmik Jondhale; *Email: [email protected]
Received: 20 Feb 2024 Revised and Accepted: 12 Apr 2025
ABSTRACT
Objective: The aim of the research is to explore the volumetric and viscometric properties of D(+)-maltose monohydrate and sodium saccharin.
Methods: These properties were assessed at temperatures between 298.15 K and 313.15 K in water and aqueous sodium saccharin (0.05, 0.15, and 0.3) mol·kg-1. The thermodynamic parameters of density (⍴) and viscosity (η), such as partial molar volume (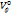 ), Masson's coefficients (
), Masson's coefficients (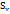 ), expansion coefficient (
), expansion coefficient (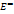 ), transfer volume (
), transfer volume (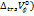 , Hepler’s Constant
, Hepler’s Constant 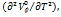 apparent specific volume (ASV), Jones-Dole B-coefficient (B),
apparent specific volume (ASV), Jones-Dole B-coefficient (B), 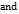 temperature-dependence dB/dT were calculated from the experimental data.
temperature-dependence dB/dT were calculated from the experimental data.
Results: The positive values of 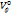 ,
,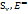 ,
,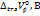 suggests that D(+)-maltose monohydrate can form structures in water and at various concentrations of aqueous sodium saccharin and increase as the concentration of the cosolute rises. Similarly, negative values of
suggests that D(+)-maltose monohydrate can form structures in water and at various concentrations of aqueous sodium saccharin and increase as the concentration of the cosolute rises. Similarly, negative values of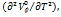 and dB/dT suggest the solute’s (D(+)-maltose monohydrate) ability to form structures with cosolute at studied concentrations.
and dB/dT suggest the solute’s (D(+)-maltose monohydrate) ability to form structures with cosolute at studied concentrations.
Conclusion: Substantial interactions between the hydrophilic groups of the solute (D(+)-maltose monohydrate) and the Na+ ion of the cosolute (sodium saccharin) were found. The studied disaccharide retained its sweetness when combined with sodium saccharin.
Keywords-D(+)-maltose monohydrate, Sodium saccharin, Density, Viscosity, Thermodynamic parameters
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2025v9i3.259 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Saccharides, or carbohydrates, are organic compounds composed of carbon, hydrogen, and oxygen, primarily serving as an energy source for muscles and the central nervous system [1-3]. They are classified based on the number of saccharide units into monosaccharides, disaccharides, and polysaccharides. Saccharides play a crucial role in biological and physiological processes, contributing to hydration properties and stability in food and medicine [4-8].
Artificial sweeteners [9-13], such as aspartame, sucralose, and saccharin, provide a low-calorie alternative to natural sugars. Sodium saccharin, which is 300 times sweeter than sucrose, is widely used in food and pharmaceutical industries due to its solubility and stability. An artificial sweetener blend with saccharides to enhance taste reduces costs and caters to diabetic patients [14-16].
The thermodynamic properties of saccharides and sweeteners, such as density and viscosity, are important for understanding their molecular interactions in aqueous solutions. These properties influence the solute-solvent interactions, impacting taste perception and structural behavior in biological and food systems [17-20]. Studies have examined how saccharides interact with water and other solutes, revealing their role in hydration, taste enhancement, and stability in various applications.
Continuation of earlier research [21, 22], this study extends the investigation to a disaccharide. The densities and viscosities were evaluated at T = (298.15-313.15) K in water and in aqueous saccharin Na salt with molalities of 0.05, 0.15, and 0.3 m. The interactions between D-(+)-maltose monohydrate-saccharin sodium salt were discovered through partial molar volumes, transfer characteristics, apparent specific volumes and B-coefficients.
MATERIALS AND METHODS
Chemicals and reagents
D-(+)-maltose monohydrate and sodium saccharin were purchased from Sigma-Aldrich with a purity of 99.0% and used without further purification.
Solution preparation
Solutions were prepared with triply distilled water in airtight glass bottles. Measurements were made using an analytical Dhona balance (±0.0001 g). Water was the solvent, and saccharide was the solute in both binary and ternary solutions, with sodium saccharin at concentrations of (0.05, 0.15, and 0.3) mol·kg-1 as the stock solution.
Physical measurement
The measurements were conducted in a glass-walled water bath with a constant temperature (±0.01 K). The densities (⍴) and viscosities (
 ) of D-(+)-maltose monohydrate in water and in aqueous sodium saccharin were measured by using a Bi-capillary Pycnometer [23-26] and Ubbelohde Viscometer [27-30] at T= (298.15-313.15) K, respectively. The Pycnometer was calibrated with organic solvents, showing good agreement with reported values. The solvent density at the studied temperature was obtained from published data [31, 32].
) of D-(+)-maltose monohydrate in water and in aqueous sodium saccharin were measured by using a Bi-capillary Pycnometer [23-26] and Ubbelohde Viscometer [27-30] at T= (298.15-313.15) K, respectively. The Pycnometer was calibrated with organic solvents, showing good agreement with reported values. The solvent density at the studied temperature was obtained from published data [31, 32].
RESULTS AND DISCUSSION
Volumetric study
At T= (298.15 K to 313.15) K, the density (⍴) of D-(+)-maltose monohydrate in aqueous sodium saccharin were studied. The apparent molar volumes ( ) of the D-(+)-maltose monohydrate were calculated using an equation [33] that relates molar mass, solution density, and molality.
) of the D-(+)-maltose monohydrate were calculated using an equation [33] that relates molar mass, solution density, and molality.
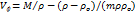 …. (1)
…. (1)
The ⍴ and  of D-(+)-maltose monohydrate in water and in aqueous sodium saccharin with molality values of 0.05, 0.15, and 0.3 were measured over temperatures from 298.15 K to 313.15 K are shown in table 1. Results showed that the densities and apparent molar volumes depend on concentration and vary linearly with solute and cosolute concentrations.
of D-(+)-maltose monohydrate in water and in aqueous sodium saccharin with molality values of 0.05, 0.15, and 0.3 were measured over temperatures from 298.15 K to 313.15 K are shown in table 1. Results showed that the densities and apparent molar volumes depend on concentration and vary linearly with solute and cosolute concentrations.
Using Masson's equation [34, 35], a relationship between  for D-(+)-maltose monohydrate in water and in aqueous sodium saccharin is presented. The partial molar volumes (
for D-(+)-maltose monohydrate in water and in aqueous sodium saccharin is presented. The partial molar volumes (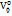 ) of D-(+)-maltose monohydrate in water matched well with published data [36] as summarized in table 2.
) of D-(+)-maltose monohydrate in water matched well with published data [36] as summarized in table 2.
 …… (2)
…… (2)
The positive 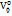 values, suggesting significant solute-solvent interactions compared to Masson's coefficients (
values, suggesting significant solute-solvent interactions compared to Masson's coefficients (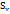 ) representing solute-solute interactions in the binary and ternary system [37, 38]. Fig. 1 show the
) representing solute-solute interactions in the binary and ternary system [37, 38]. Fig. 1 show the 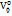 obtained at experimental temperature for 0.05 m sodium saccharin.
obtained at experimental temperature for 0.05 m sodium saccharin.
Table 1: Densities (ρ/kg·m-3), and apparent molar volumes (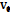 ) of (D-(+)-maltose monohydrate) in water and aqueous solutions of (0.05, 0.15, and 0.3) m Na saccharin at T = (298.15-313.15) K
) of (D-(+)-maltose monohydrate) in water and aqueous solutions of (0.05, 0.15, and 0.3) m Na saccharin at T = (298.15-313.15) K
m (mol·kg-1) |
(T/K) | |||||||
| 298.15 | 303.15 | 308.15 | 313.15 | |||||
| ⍴ | 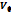 ·106 ·106 |
⍴ | 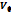 ·106 ·106 |
⍴ | 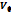 ·106 ·106 |
⍴ | 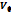 ·106 ·106 |
|
| (kg⋅m-3) | (m3·mol-1) | (kg⋅m-3) | (m3⋅mol-1) | (kg⋅m-3) | (m3⋅mol-1) | (kg⋅m-3) | (m3⋅mol-1) | |
| D-(+)-maltose monohydrate+water | ||||||||
| 0.0000 | 997.05 | 995.65 | 994.03 | 992.22 | ||||
| 0.0405 | 1002.35 | 228.52 | 1000.92 | 229.41 | 999.28 | 230.07 | 997.45 | 230.88 |
| 0.0806 | 1007.48 | 228.81 | 1006.02 | 229.71 | 1004.35 | 230.50 | 1002.49 | 231.32 |
| 0.1205 | 1012.48 | 229.02 | 1010.97 | 230.09 | 1009.27 | 230.94 | 1007.40 | 231.63 |
| 0.1606 | 1017.38 | 229.36 | 1015.80 | 230.52 | 1014.12 | 231.20 | 1012.21 | 232.03 |
| 0.2005 | 1022.17 | 229.56 | 1020.54 | 230.89 | 1018.81 | 231.62 | 1016.89 | 232.38 |
| D-(+)-maltose monohydrate+0.05 m Na saccharin | ||||||||
| 0.0000 | 1001.20 | 999.69 | 998.07 | 997.50 | ||||
| 0.0399 | 1006.39 | 229.00 | 1004.85 | 229.81 | 1003.20 | 230.75 | 1002.60 | 231.57 |
| 0.0796 | 1011.44 | 229.20 | 1009.88 | 230.05 | 1008.19 | 231.04 | 1007.56 | 231.86 |
| 0.1199 | 1016.45 | 229.53 | 1014.86 | 230.36 | 1013.14 | 231.37 | 1012.47 | 232.28 |
| 0.1599 | 1021.31 | 229.80 | 1019.69 | 230.65 | 1018.40 | 231.79 | 1017.22 | 232.67 |
| 0.1998 | 1026.06 | 230.02 | 1024.90 | 230.99 | 1022.58 | 232.13 | 1021.86 | 232.97 |
| D-(+)-maltose monohydrate+0.15 m Na saccharin | ||||||||
| 0.0000 | 1008.57 | 1007.01 | 1005.27 | 1003.39 | ||||
| 0.0405 | 1013.79 | 229.38 | 1012.21 | 230.15 | 1010.44 | 230.95 | 1008.76 | 231.78 |
| 0.0800 | 1018.76 | 229.65 | 1017.15 | 230.43 | 1015.35 | 231.36 | 1013.42 | 232.18 |
| 0.1199 | 1023.68 | 229.95 | 1022.03 | 230.86 | 1020.21 | 231.71 | 1018.25 | 232.58 |
| 0.1598 | 1028.45 | 230.41 | 1026.79 | 231.21 | 1024.93 | 232.15 | 1022.96 | 232.92 |
| 0.1998 | 1033.12 | 230.81 | 1031.44 | 231.58 | 1029.55 | 232.53 | 1027.55 | 233.34 |
| D-(+)-maltose monohydrate+0.3 m Na saccharin | ||||||||
| 0.0000 | 1021.10 | 1018.32 | 1016.82 | 1014.79 | ||||
| 0.0401 | 1026.20 | 229.74 | 1023.39 | 230.71 | 1021.86 | 231.69 | 1019.80 | 232.59 |
| 0.0805 | 1031.21 | 230.08 | 1028.39 | 230.97 | 1026.81 | 232.04 | 1024.72 | 232.99 |
| 0.1208 | 1036.10 | 230.39 | 1033.23 | 231.41 | 1031.62 | 232.47 | 1029.51 | 233.35 |
| 0.1602 | 1040.75 | 230.78 | 1037.85 | 231.82 | 1036.21 | 232.84 | 1034.07 | 233.78 |
| 0.2004 | 1045.38 | 231.17 | 1042.45 | 232.21 | 1040.77 | 233.27 | 1038.83 | 234.18 |
Partial molar volume,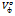 are related to temperature as
are related to temperature as
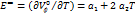 …… (3)
…… (3)
In the above equation a1, and a2 are constants. The expansion coefficient 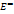 can be calculated using the above formula.
can be calculated using the above formula.
As the temperature rises, 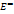 values increase for the given system. The
values increase for the given system. The 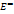 outcomes are positive and rise in accordance with increasing cosolute concentration [39] as shown in table 2, suggesting the stronger solute-solvent interactions.
outcomes are positive and rise in accordance with increasing cosolute concentration [39] as shown in table 2, suggesting the stronger solute-solvent interactions.
Partial molar volume of transfer at infinite dilution (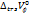 ) is plotted against the molality of sodium saccharin for each studied temperature, suggesting an increase with higher co-solute concentrations [40]. The interactions between D-maltose and aqueous sodium saccharin can be classified into two types:
) is plotted against the molality of sodium saccharin for each studied temperature, suggesting an increase with higher co-solute concentrations [40]. The interactions between D-maltose and aqueous sodium saccharin can be classified into two types:
I) Hydrophilic-ionic interaction: These take place between cosolute ion and hydrophilic groups of maltose (-C=O,-OH, and -O-).
II) Hydrophobic-ionic interaction: These take place between the cosolute ion and the hydrophobic group of maltose.
The "co-sphere overlap model"[41-43] states that type (I) interactions positively contribute to the transfer volume (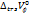 ) while type (II) interactions negatively contribute to it. The positive values obtained for the studied system suggest that the type (I) interactions predominate over type (II).
) while type (II) interactions negatively contribute to it. The positive values obtained for the studied system suggest that the type (I) interactions predominate over type (II).
Hepler’s constant 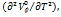 data for the studied system are tabulated in table 2 are positive, indicating solute's ability to build structure and it decrease with the increasing sodium saccharin concentration [44, 45].
data for the studied system are tabulated in table 2 are positive, indicating solute's ability to build structure and it decrease with the increasing sodium saccharin concentration [44, 45].
Aqueous solutions are categorized into salt, sweet, bitter, and sour based on taste, as classified by Shamil and Birch [46]. The apparent specific volume (ASV) of solutes in solvent and cosolute is calculated using the equation ASV =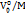 . According to Parke et al. [47], the ASV range for sweet molecules is in the range (0.51 to 0.71) X 10-6 m3. kg-1, with an ideal value of 0.618×10⁻⁶ m³·kg⁻¹. The table 2 shows the ASV values for studied system. For maltose, the ASV values ranged from 0.634 to 0.644×10-6 m3. kg-1. The maltose retained their sweetness when mixed with sodium saccharin stock solutions.
. According to Parke et al. [47], the ASV range for sweet molecules is in the range (0.51 to 0.71) X 10-6 m3. kg-1, with an ideal value of 0.618×10⁻⁶ m³·kg⁻¹. The table 2 shows the ASV values for studied system. For maltose, the ASV values ranged from 0.634 to 0.644×10-6 m3. kg-1. The maltose retained their sweetness when mixed with sodium saccharin stock solutions.

Fig. 1: Variation of 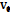 (m3·mol-1) of D-(+)-maltose monohydrate in sodium saccharin with molality 0.05 m (mol·kg-1)
(m3·mol-1) of D-(+)-maltose monohydrate in sodium saccharin with molality 0.05 m (mol·kg-1)
Table 2:  ), (
), ( ),
), 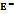 , ASV,
, ASV, , and
, and  ) of disaccharide, D-(+)-maltose monohydrate in water and aqueous Na saccharin (0.05, 0.15, and 0.3) m (mol·kg-1) at T = (298.15, 303.15, 308.15, and 313.15) K
) of disaccharide, D-(+)-maltose monohydrate in water and aqueous Na saccharin (0.05, 0.15, and 0.3) m (mol·kg-1) at T = (298.15, 303.15, 308.15, and 313.15) K
| System | Parameters | (T/K) | |||
| 298.15 | 303.15 | 308.15 | 313.15 | ||
| D-(+)-maltose monohydrate+water | 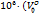 ) ) |
228.262 | 228.985 | 229.717 | 230.526 |
106 ⋅ (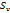 ) ) |
6.58 | 9.44 | 9.52 | 9.30 | |
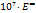 |
0.1376 | 0.1462 | 0.1547 | 0.1633 | |
| 106⋅ASV | 0.634 | 0.636 | 0.638 | 0.640 | |
 |
0.001715 | ||||
D-(+)-maltose monohydrate + 0.05 m Na saccharin |
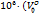 ) ) |
228.723 | 229.482 | 230.363 | 231.188 |
106 ⋅ (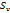 ) ) |
6.560 | 7.410 | 8.793 | 9.010 | |
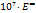 |
0.1555 | 0.1622 | 0.1688 | 0.1755 | |
| 106⋅ASV | 0.635 | 0.637 | 0.639 | 0.642 | |
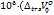 ) ) |
0.460 | 0.497 | 0.645 | 0.662 | |
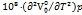 |
0.001329 | ||||
D-(+)-maltose monohydrate+ 0.15 m Na saccharin |
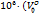 ) ) |
228.949 | 229.749 | 230.544 | 231.397 |
106 ⋅ (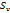 ) ) |
9.10 | 9.13 | 9.94 | 9.68 | |
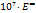 |
0.1550 | 0.1602 | 0.1654 | 0.1707 | |
| 106⋅ASV | 0.635 | 0.638 | 0.640 | 0.642 | |
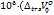 ) ) |
0.686 | 0.764 | 0.827 | 0.872 | |
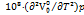 |
0.001047 | ||||
D-(+)-maltose monohydrate+ 0.3 m Na saccharin |
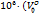 ) ) |
229.362 | 230.264 | 231.272 | 232.184 |
106 ⋅ (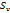 ) ) |
8.88 | 9.63 | 9.89 | 9.92 | |
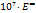 |
0.1880 | 0.1889 | 0.1899 | 0.1909 | |
| 106⋅ASV | 0.637 | 0.639 | 0.642 | 0.644 | |
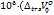 ) ) |
1.100 | 1.279 | 1.554 | 1.658 | |
 |
0.000197 | ||||
Volumetric study
The viscosities (η) of D-(+)-maltose monohydrate in water and aqueous sodium saccharin were measured at temperatures (298.15, 303.15, 308.15, and 313.15) K and concentrations (m = 0.05, 0.15, and 0.30). The result indicates that viscosity decreases with increasing temperature but increases as the co-solute concentration (sodium saccharin) rises. The relative viscosities (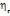 ) were calculated by comparing the viscosity in the ternary solution, η (maltose+sodium saccharin+water), to that in the solvent mixture (sodium saccharin+water)
) were calculated by comparing the viscosity in the ternary solution, η (maltose+sodium saccharin+water), to that in the solvent mixture (sodium saccharin+water)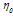 , as shown in table 3. These values were then used to calculate B-coefficients using the Jones-Dole equation [48-50] as:
, as shown in table 3. These values were then used to calculate B-coefficients using the Jones-Dole equation [48-50] as:
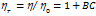 …… (5)
…… (5)
The B-coefficients, which characterize solute-solvent interactions, were determined from the slope of relative viscosity versus molality plots. The B-coefficients for the studied system at the different temperatures showed a decrease as temperature increased (table 4), suggesting the solute (maltose) contributes to structure formation due to the ordered hydrogen-bonded structure around it [51]. Fig. 2 shows the variation of relative viscosity,  of maltose monohydrate in Na saccharin with molality 0.05 m (mol·kg-1). Similar results were obtained for (0.15 and 0.30) m sodium saccharin. Positive B-coefficient values indicate stronger solute-solvent interactions compared to solute-solute interactions. The results also provide insight into the ion solvation behaviour and the ability of solutes to form structures.
of maltose monohydrate in Na saccharin with molality 0.05 m (mol·kg-1). Similar results were obtained for (0.15 and 0.30) m sodium saccharin. Positive B-coefficient values indicate stronger solute-solvent interactions compared to solute-solute interactions. The results also provide insight into the ion solvation behaviour and the ability of solutes to form structures.
The dB/dT ratio is an important factor in determining a solute's capacity to form or break structures derived from temperature-dependent B-coefficients [52]. Table 4 shows the dB/dT values for the studied saccharide in both water and aqueous sodium saccharin at studied concentrations and temperatures. The negative dB/dT values observed for studied systems suggest that the solute is more likely to form structures [53].

Fig. 2: Variation of relative viscosity,  of maltose monohydrate in Na saccharin with molality 0.05 m (mol·kg-1)
of maltose monohydrate in Na saccharin with molality 0.05 m (mol·kg-1)
Table 3: Viscosities (η/mPa⋅s), and relative viscosities (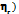 of disaccharide, D-(+)-maltose monohydrate in H2O and aqueous solutions of (0.05, 0.15, and 0.3) m Na saccharin at T = (298.15, 303.15, 308.15, and 313.15) K
of disaccharide, D-(+)-maltose monohydrate in H2O and aqueous solutions of (0.05, 0.15, and 0.3) m Na saccharin at T = (298.15, 303.15, 308.15, and 313.15) K
m (mol⋅kg-1) |
η | 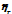 |
||||||
| (T/K) | ||||||||
| 298.15 | 303.15 | 308.15 | 313.15 | 298.15 | 303.15 | 308.15 | 313.15 | |
| D-(+)-maltose monohydrate+H2O | ||||||||
| 0.0000 | 0.8900 | 0.7972 | 0.7191 | 0.6527 | 0.9898 | 0.9916 | 0.9906 | 0.9903 |
| 0.0405 | 0.9243 | 0.8279 | 0.7449 | 0.6757 | 1.0385 | 1.0386 | 1.0358 | 1.0352 |
| 0.0806 | 0.9594 | 0.8583 | 0.7736 | 0.7019 | 1.0780 | 1.0767 | 1.0758 | 1.0754 |
| 0.1205 | 0.9995 | 0.8928 | 0.8020 | 0.7273 | 1.1230 | 1.1200 | 1.1153 | 1.1142 |
| 0.1606 | 1.0377 | 0.9260 | 0.8331 | 0.7543 | 1.1660 | 1.1615 | 1.1585 | 1.1556 |
| 0.2005 | 1.0845 | 0.9664 | 0.8675 | 0.7870 | 1.2186 | 1.2122 | 1.2063 | 1.2057 |
| D-(+)-maltose monohydrate+0.05 m Na saccharin | ||||||||
| 0.0000 | 0.9071 | 0.8040 | 0.7187 | 0.6582 | 0.9907 | 0.9900 | 0.9906 | 0.9923 |
| 0.0399 | 0.9421 | 0.8328 | 0.7446 | 0.6814 | 1.0386 | 1.0358 | 1.0360 | 1.0353 |
| 0.0796 | 0.9795 | 0.8660 | 0.7735 | 0.7093 | 1.0798 | 1.0771 | 1.0762 | 1.0776 |
| 0.1199 | 1.0213 | 0.9026 | 0.8053 | 0.7380 | 1.1259 | 1.1226 | 1.1205 | 1.1212 |
| 0.1599 | 1.0644 | 0.9383 | 0.8393 | 0.7653 | 1.1734 | 1.1670 | 1.1677 | 1.1627 |
| 0.1998 | 1.1071 | 0.9754 | 0.8693 | 0.7945 | 1.2204 | 1.2132 | 1.2095 | 1.2071 |
| D-(+)-maltose monohydrate+0.15 m Na saccharin | ||||||||
| 0.0000 | 0.9309 | 0.8372 | 0.7455 | 0.6772 | 0.9952 | 0.9938 | 0.9908 | 0.9924 |
| 0.0405 | 0.9734 | 0.8734 | 0.7747 | 0.7030 | 1.0456 | 1.0433 | 1.0391 | 1.0381 |
| 0.0800 | 1.0135 | 0.9069 | 0.8042 | 0.7303 | 1.0887 | 1.0832 | 1.0787 | 1.0783 |
| 0.1199 | 1.0572 | 0.9457 | 0.8402 | 0.7589 | 1.1357 | 1.1296 | 1.1270 | 1.1207 |
| 0.1598 | 1.0995 | 0.9820 | 0.8698 | 0.7854 | 1.1811 | 1.1730 | 1.1667 | 1.1598 |
| 0.1998 | 1.1500 | 1.0266 | 0.9095 | 0.8209 | 1.2354 | 1.2263 | 1.2200 | 1.2121 |
| D-(+)-maltose monohydrate+0.3 m Na saccharin | ||||||||
| 0.0000 | 0.9738 | 0.8722 | 0.7817 | 0.7099 | 0.9969 | 0.9953 | 0.9944 | 0.9938 |
| 0.0401 | 1.0195 | 0.9112 | 0.8157 | 0.7383 | 1.0469 | 1.0447 | 1.0435 | 1.0399 |
| 0.0805 | 1.0645 | 0.9488 | 0.8477 | 0.7674 | 1.0932 | 1.0878 | 1.0843 | 1.0810 |
| 0.1208 | 1.1108 | 0.9892 | 0.8824 | 0.7991 | 1.1406 | 1.1341 | 1.1288 | 1.1256 |
| 0.1602 | 1.1600 | 1.0311 | 0.9203 | 0.8311 | 1.1912 | 1.1822 | 1.1773 | 1.1706 |
| 0.2004 | 1.2071 | 1.0743 | 0.9580 | 0.8632 | 1.2396 | 1.2317 | 1.2255 | 1.2158 |
Table 4: Viscosity B-coefficients, and dB/dT, of disaccharide, D-(+)-maltose monohydrate in water and aqueous solutions of (0.05, 0.15, and 0.3) m Na saccharin at T = (298.15, 303.15, 308.15, and 313.15) K
| System | Parameters | (T/K) | |||
| 298.15 | 303.15 | 308.15 | 313.15 | ||
D-(+)-maltose monohydrate+ water |
B | 1.120 | 1.080 | 1.059 | 1.053 |
| dB/dT | -0.0044 | ||||
D-(+)-maltose monohydrate+ 0.05 m Na saccharin |
B | 1.143 | 1.111 | 1.096 | 1.072 |
| dB/dT | -0.0046 | ||||
D-(+)-maltose monohydrate+ 0.15 m Na saccharin |
B | 1.185 | 1.144 | 1.129 | 1.078 |
| dB/dT | -0.0067 | ||||
D-(+)-maltose monohydrate+ 0.3 m Na saccharin |
B | 1.208 | 1.170 | 1.142 | 1.103 |
| dB/dT | -0.0068 | ||||
CONCLUSION
The study explores the use of a blend of D(+)-maltose monohydrate and sodium saccharin as an alternative to sweeteners to improve taste and stability in sweetened compositions. The volumetric and viscometric properties of D(+)-maltose monohydrate in water and aqueous sodium saccharin were measured at temperatures ranging from 298.15 K to 313.15 K. The thermodynamic parameters, such as  ,
, 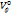 ,
,  ,
,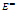 ,
, 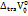 , ASV, B,
, ASV, B,  dB/dT were calculated from the experimental data. The findings suggest that transfer occurs from water to aqueous sodium saccharin, with the transfer volume increasing as the concentration of the cosolute rises. Additionally, the sweetness of the studied disaccharide was preserved (0.634-0.644)10-6. m3. kg-1 when blended with sodium saccharin. The solute (D(+)-maltose monohydrate) can form structures in water and at various concentrations of aqueous sodium saccharin system, as shown by positive B-coefficient and negative dB/dT values.
dB/dT were calculated from the experimental data. The findings suggest that transfer occurs from water to aqueous sodium saccharin, with the transfer volume increasing as the concentration of the cosolute rises. Additionally, the sweetness of the studied disaccharide was preserved (0.634-0.644)10-6. m3. kg-1 when blended with sodium saccharin. The solute (D(+)-maltose monohydrate) can form structures in water and at various concentrations of aqueous sodium saccharin system, as shown by positive B-coefficient and negative dB/dT values.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Prajakta Jondhale: Interpretation of data, and Drafting of the manuscript. Valmik Jondhale: Analysis of the results and final manuscript.
CONFLICT OF INTERESTS
The authors declare no conflict of interest among the author.
REFERENCES
Caffall KH, Mohnen D. The structure, function and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res. 2009;344(14):1879-900. doi: 10.1016/j.carres.2009.05.021, PMID 19616198.
Franks F. Physical chemistry of small carbohydrates equilibrium solution properties. Pure Appl Chem. 1987;59(9):1189-202. doi: 10.1351/pac198759091189.
Birch GG, Pepper T. Protection of vitamin C by sugars and their hydrogenated derivatives. J Agric Food Chem. 1983;31(5):980-5. doi: 10.1021/jf00119a015.
Angyal SJ. Complexes of carbohydrates with metal cations. I. determination of the extent of complexing by NMR spectroscopy. Aust J Chem. 1972;25(9):1957-66. doi: 10.1071/CH9721957.
Kaiwar SP, Rao CP. Soluble complexes of early first-row transition metal ions with D-glucose. Carbohydr Res. 1992 Dec 31;237:203-10. doi: 10.1016/S0008-6215(92)84244-M.
Tajmir Riahi HA. Interaction of D-glucose with alkaline earth metal ions synthesis, spectroscopic and structural characterization of Mg(II) and Ca(II)-D-glucose adducts and the effect of metal ion binding on anomeric configuration of the sugar. Carbohydr Res. 1988;183(1):35-46. doi: 10.1016/0008-6215(88)80043-0, PMID 3233597.
Lichtenthaler FW. Towards improving the utility of ketoses as organic raw materials. Carbohydr Res. 1998;313(2):69-89. doi: 10.1016/S0008-6215(98)00222-5.
Dey S, Rahman M, Islam M, Dutta S, Hossain M, Dhar P. Studies on volumetric and viscometric properties of L-glutamic acid in aqueous solution of glucose over a range of temperatures (298K to 323K). Lett Appl Nanobiosci. 2020;9(4):1547-61. doi: 10.33263/LIANBS94.15471561.
Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners a review. J Food Sci Technol. 2014;51(4):611-21. doi: 10.1007/s13197-011-0571-1, PMID 24741154.
Harned HS, Owen BB. The physical chemistry of electrolytic solutions. ACS Monogr No. 137. 3rd ed; 1958.
Grenby TH, Parker KJ, Lindley MG. Developments in sweeteners-2. Elsevier; 1983.
Poshala K. Int J Eng Sci Comput. 2020;10:27416.
Periyasamy A. Artificial sweeteners. Int J Res Rev. 2019;6(1):120-8.
Nabors LO B, Gelardi RC. Alternative sweeteners. 2nd ed. New York: Marcel Dekker Inc; 1991.
Kretchmer N, Hollenbeck C. Sugars and sweeteners. Boca Raton: CRC Press; 1991.
Wani MM, Bhat TA. Sugar substitutes and artificial sweeteners. JMS SKIMS. 2019;22(1):90. doi: 10.33883/jms.v22i1.439.
Dey S, Rahman M, Islam M, Dutta S, Hossain M, Dhar P. Studies on volumetric and viscometric properties of L-glutamic acid in aqueous solution of glucose over a range of temperatures (298K to 323K). Lett Appl Nanobiosci. 2020;9(4):1547-61. doi: 10.33263/LIANBS94.15471561.
Kaur K, Arti S, Ghosh TK, Banipal TS, Banipal PK. To study the interactions between saccharide/their derivatives and bactericidal cefadroxil drug: volumetric acoustic and molecular docking studies. J Chem Thermodyn. 2021 Aug;159:106477. doi: 10.1016/j.jct.2021.106477.
Amirchand KD, Kaur S, Banipal TS, Singh V. Volumetric and 1H NMR spectroscopic studies of saccharides calcium lactate interactions in aqueous solutions. J Mol Liq. 2021 Jul 15;334:116077. doi: 10.1016/j.molliq.2021.116077.
Sharma M, Banipal PK, Banipal TS. Hydration characteristics structural effects and the taste quality of some polyhydroxy compounds in aqueous solutions of nicotinic acid (vitamin B3) at (288.15-318.15) K. Food Chem. 2020 Apr 25;310:125861. doi: 10.1016/j.foodchem.2019.125861, PMID 31767485.
Kharat SJ, Jondhale VR. Volumetric study of saccharide interactions (D-arabinose, D-xylose, and D-galactose) in sodium saccharin at 298.15 K. JASR. 2021;12(3):152-9. doi: 10.55218/JASR.202112322.
Kharat SJ, Jondhale VR. Volumetric study of monosaccharides (D-ribose and D-mannose) saccharin sodium salt in aqueous solutions at T = 298.15 K. IJFANS. 2022 Dec 3;11:3413-23.
Robertson GR. A graduated pycnometer. Ind Eng Chem Anal Ed. 1939;11(8):464. doi: 10.1021/ac50136a025.
Parker HC, Parker EW. Chloride solutions as determined with a new pycnometer. J Phys Chem. 1925;29(2):130-7. doi: 10.1021/j150248a002.
Kharat SJ, Nikam PS. Density and viscosity studies of binary mixtures of aniline+benzene and ternary mixtures of (aniline+Benzene+N,N-Dimethylformamide) at 298.15, 303.15, 308.15, and 313.15 K. J Mol Liq. 2007 Mar 15;131-132:81-6. doi: 10.1016/j.molliq.2006.08.053.
Kharat SJ. Density viscosity and ultrasonic velocity studies of aqueous solutions of sodium salycilate and its hydration free energy. Phys Chem Liq. 2014;52(1):7-16. doi: 10.1080/00319104.2013.795856.
Swindells JF. Physical methods of organic chemistry weissberger a editor. Part I. New York: Interscience; 1959. p. 689.
Subha MC, Rao SB. Densities and viscosities of propionic acid in benzene methylbenzene ethylbenzene and propylbenzene. J Chem Eng Data. 1988;33(4):404-6. doi: 10.1021/je00054a005.
Sathyanarayana B, Ranjithkumar B, Savitha Jyostna T, Satyanarayana N. Densities and viscosities of binary liquid mixtures of N-Methylacetamide with some chloroethanes and chloroethenes at T=308.15 K. J Chem Thermodyn. 2007;39(1):16-21. doi: 10.1016/j.jct.2006.06.009.
Kharat SJ. Partial molar volume jones dole coefficient and limiting molar isentropic compressibility of sodium ibuprofen in water and its hydration number and hydration free energy. Thermochim Acta. 2013 Aug 20;566:124-9. doi: 10.1016/j.tca.2013.05.030.
Lide DR. CRC handbook of chemistry and physics. 73rd ed. Boca Raton FL: CRC Press; 1992.
Engineering tool box H2O density specific weight and thermal expansion coefficient; 2003. Available from: https://www.engineeringtoolbox.com/H2O-density-specific-weight-d_595.html.
Kupke DW. Physical principles and techniques of physical chemistry part-C. New York: Academic press; 1973.
Masson DO. XXVIII. Solute molecular volumes in relation to solvation and ionization. Lond Edinb Dublin Philos Mag J Sci. 1929;8(49):218-35. doi: 10.1080/14786440808564880.
Ali A, Bidhuri P, Malik NA, Uzair S. Density viscosity and refractive index of mono di and tri-saccharides in aqueous glycine solutions at different temperatures. Arab J Chem. 2019;12(7):1684-94. doi: 10.1016/j.arabjc.2014.08.027.
Banipal PK, Banipal TS, Ahluwalia JC, Lark BS. Partial molar heat capacities and volumes of transfer of some saccharides from water to aqueous urea solutions at T=298.15 K. J Chem Thermodyn. 2000;32(10):1409-32. doi: 10.1006/jcht.2000.0689.
Franks F, Water A. Comprehensive treatise: volume 4. Aqueous solutions of amphiphiles and macromolecules; water. Springer; 1975.
Franks F, Quickenden MA, Reid DS, Watson B. Calorimetric and volumetric studies of dilute aqueous solutions of cyclic ether derivatives. Trans Faraday Soc. 1970;66:582-9. doi: 10.1039/tf9706600582.
Nain AK, Chand D. Volumetric ultrasonic and viscometric behaviour of glycine Dl-alanine and l-valine in aqueous 1,4-butanediol solutions at different temperatures. J Chem Thermodyn. 2009;41(2):243-9. doi: 10.1016/j.jct.2008.09.008.
Banipal PK, Singh V, Banipal TS. Effect of sodium acetate on the volumetric behaviour of some mono di and tri-saccharides in aqueous solutions over temperature range (288.15 to 318.15) K. J Chem Thermodyn. 2010;42(1):90-103. doi: 10.1016/j.jct.2009.07.015.
Freidman HL, Krishnan CV. In: Franks F, Editors, Chapter 1. Water: a comprehensive Treatise. New York: Plenum press; 1993.
Gurney RW. Ionic processes in solution. New York: McGraw Hill; 1953.
Desnoyers JE, Arel M, Perron G, Jolicoeur C. Apparent molal volumes of alkali halides in water at 25˚. Influence of structural hydration interactions on the concentration dependence. J Phys Chem. 1969;73(10):3346-51. doi: 10.1021/j100844a032.
Hepler LG. Thermal expansion and structure in water and aqueous solutions. Can J Chem. 1969;47(24):4613-7. doi: 10.1139/v69-762.
Roy MN, Das RK, Bhattacharjee A. Apparent molar volume viscosity B-coefficient and adiabatic compressibility of tetrabutylammonium bromide in aqueous ascorbic acid solutions at T = 298.15, 308.15, and 318.15 K. Russ J Phys Chem. 2010;84(13):2201-10. doi: 10.1134/S0036024410130017.
Shamil S, Birch GG. A conceptual model of taste receptors. Endeavour. 1990;14(4):191-3. doi: 10.1016/0160-9327(90)90043-q, PMID 1706257.
Parke SA, Birch GG, Portmann MO, Kilcast DA. A study of the solution properties of selected binary mixtures of bulk and intense sweeteners in relation to their psychophysical characteristics. Food Chem. 1999;67(3):247-59. doi: 10.1016/S0308-8146(99)00125-9.
Jones G, Dole M. The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J Am Chem Soc. 1929;51(10):2950-64. doi: 10.1021/ja01385a012.
Pande R. Partial molar volumes and viscosity b-coefficient of n-phenylbenzohydroxamic acid in dimethylsulfoxide at different temperatures. J Chem Eng Data. 2008;53(7):1458-61. doi: 10.1021/je7006956.
Kaminsky M. Ion solvent interaction and the viscosity of strong electrolyte solutions. Discuss Faraday Soc. 1957;24(0):171-9. doi: 10.1039/df9572400171.
Marcus Y. Viscosity B-coefficients structural entropies and heat capacities and the effects of ions on the structure of water. J Solut Chem. 1994;23(7):831-48. doi: 10.1007/BF00972677.
Feakins D, Freemantle DJ, Lawrence KG. Transition state treatment of the relative viscosity of electrolytic solutions. Applications to aqueous non-aqueous and methanol + water systems. J Chem Soc Faraday Trans 1. 1974;70. doi: 10.1039/f19747000795.
Out DJ, Los JM. Viscosity of aqueous solutions of univalent electrolytes from 5 to 95 °C. J Solut Chem. 1980;9(1):19-35. doi: 10.1007/BF00650134.